CLINICAL PHARMACOLOGY
Mechanism of Action
Remdesivir is an inhibitor of the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), which is essential for viral replication. Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in delayed chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.
Pharmacokinetics
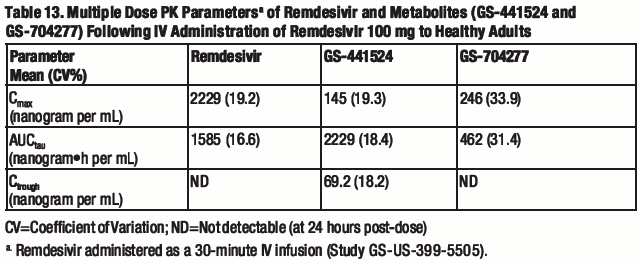
The pharmacokinetic (PK) properties of remdesivir and metabolites have been evaluated in adults in several Phase 1 trials and are provided in Table 12. The multiple dose PK parameters of remdesivir and metabolites in healthy adults are provided in Table 13.
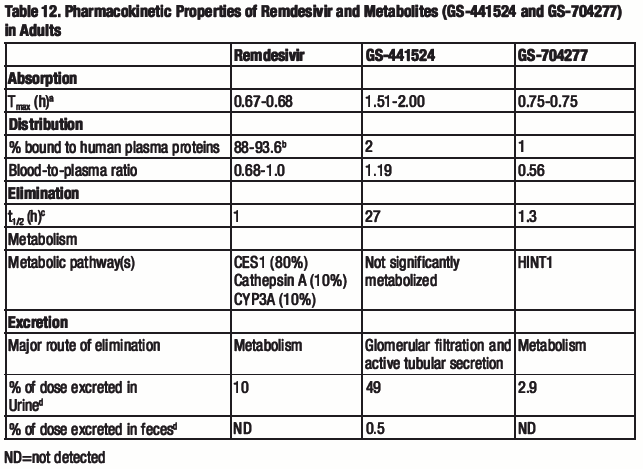
a. Remdesivir administered as a 30-minute IV infusion (Study GS-US-399-5505); range of median observed on Day 1 and Day 5 or 10.
b. Range of protein binding for remdesivir from 2 independent experiments show no evidence of concentration dependent protein binding for remdesivir.
c. Median (Study GS-US-399-4231).
d. Mean (Study GS-US-399-4231).
Specific Populations
Sex, Race and Age
Pharmacokinetic differences based on sex, race, and age have not been evaluated.
Pediatric Patients
The pharmacokinetics of remdesivir in pediatric patients has not been evaluated.
PBPK modeling of pharmacokinetic data from healthy adults was used to derive pediatric doses. PBPK modeling incorporated in vitro data for remdesivir and other similar compounds along with age-dependent changes in physiology (e.g., organ volume/function, blood flow), metabolism, distribution, and elimination of remdesivir. Pediatric doses are expected to result in comparable steady-state exposures of remdesivir and metabolites as observed in healthy adults following administration of the recommended dosage regimen.
Renal Impairment
Because the excipient SBECD is renally cleared and accumulates in patients with decreased renal function, administration of drugs formulated with SBECD (such as remdesivir) is not recommended in adult and pediatric patients (>28 days old) with eGFR less than 30 mL per minute or in full-term neonates (≥7 days and ≤28 days old) with serum creatinine clearance ≥1 mg/dL unless the potential benefit outweighs the potential risk.


