DOSAGE AND ADMINISTRATION
General Information
- The optimal dosing and duration of treatment are unknown. The suggested dose and duration may be updated as data from clinical trials becomes available.
- Adult and pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing of remdesivir and during treatment as clinically appropriate.[see Dosage and Administration] & [Use in Specific Populations].
- Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir. [see Warnings and Precautions] & [Use in Specific Populations].
- Determine prothrombin time in all patients before starting Remdesivir and monitor while receiving remdesivir as clinically appropriate. [see Clinical Trials Experience]
- Remdesivir should be administered via intravenous (IV) infusion only. Do not administer as an intramuscular (IM) injection.
- Extension of administration of drug beyond 5 days to 10 days is not recommended.
Adult Patients
- The dose of the drug for adults should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
- Remdesivir is to be administered via intravenous infusion in a total volume of up to 250 mL 0.9% saline over 30 to 120 minutes [For further information see Dosage and Administration].
- Extension of administration of the drug beyond 5 days to 10 days is not recommended.
All adult patients must have creatinine clearance determined before dosing [For further information see Dosage and Administration].
Hepatic laboratory testing should be performed in all patients starting remdesivir and daily while receiving remdesivir dosing [For further information see Dosage and Administration].
Pediatric Patients
Dosing in pediatric patients is based upon physiologically based (PBPK) modeling and simulation of pharmacokinetic data from healthy adult subjects. The recommended pediatric dose for pediatric patients weighing between 3.5 kg and <40 kg should be calculated using the mg/kg dose according to the patient’s weight [For further information see Dosage and Administration];
- The dose of the drug for pediatric patients weighing more than 40 kg should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
- The dose for pediatric patients with bodyweight between 3.5 kg and less than 40 kg should be a single dose of 5 mg/kg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 2.5 mg/kg, infused intravenously over 30-120 minutes for 4 days.
- Extension of administration of the drug beyond 5 days to 10 days is not recommended.
Pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing [For further information see Dosage and Administration].
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir dosing [For further information see Dosage and Administration].
Pregnancy
Remdesivir should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus.
Renal Impairment
Use in patients with renal impairment is based on potential risk and potential benefit considerations. Patients with eGFR greater than or equal to 30 mL/min are reported to have received remdesivir for treatment of COVID-19 with no dose adjustment of remdesivir. All patients must have an eGFR determined before dosing.
Because the excipient sulfobutylether-β-cyclodextrin sodium salt (SBECD) is renally cleared and accumulates in patients with decreased renal function, administration of drugs formulated with SBECD (such as remdesivir) is not recommended in adults and pediatric patients (>28 days old) with eGFR less than 30 mL per minute or in full-term neonates (≥7 days and ≤28 days old) with serum creatinine clearance ≥1 mg/dL unless the potential benefit outweighs the potential risk.
Hepatic Impairment
The pharmacokinetics of remdesivir have not been evaluated in patients with hepatic impairment. It is not known if a dosage adjustment is needed in patients with hepatic impairment and remdesivir should only be used in patients with hepatic impairment if the potential benefit outweighs the potential risk [For further information see Warnings and Precautions].
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir.
Adult Dose Preparation and Administration Remdesivir for Injection, 100 mg, Lyophilized Powder
Reconstitution Instructions
-
Remove the required number of single-dose vial(s) from storage. For each vial:
- Aseptically reconstitute remdesivir lyophilized powder by addition of 19 mL of sterile water for injection using a suitably sized syringe and needle per vial.
- Discard the vial if a vacuum does not pull the sterile water for injection into the vial.
- Immediately shake the vial for 30 seconds.
- Allow the contents of the vial to settle for 2 to 3 minutes. A clear solution should result.
- If the contents of the vial are not completely dissolved, shake the vial again for 30 seconds and allow the contents to settle for 2 to 3 minutes. Repeat this procedure as necessary until the contents of the vial are completely dissolved.
- Following reconstitution, each vial contains 100 mg/20 mL (5 mg/mL) of remdesivir solution.
- Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit.
- After reconstitution, the total storage time before administration should not exceed 4 hours at room temperature or 24 hours at refrigerated temperature (2°C to 8°C [36°F to 46°F]).
Dilution Instructions
Care should be taken during admixture to prevent inadvertent microbial contamination. As there is no preservative or bacteriostatic agent present in this product, aseptic technique must be used in the preparation of the final parenteral solution. It is always recommended to administer IV medication immediately after preparation when possible.
-
Using Table 1, determine the volume of 0.9% saline to withdraw from the infusion bag.
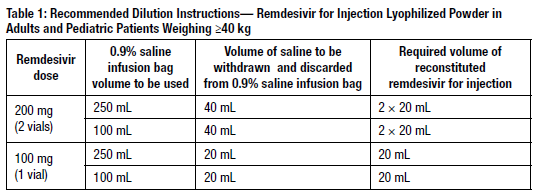
- Withdraw the required volume of saline from the bag using an appropriately sized syringe and needle. Discard the saline that was withdrawn from the bag.
- Withdraw the required volume of reconstituted remdesivir for injection from the remdesivir vial using an appropriately sized syringe per Table 1. Discard any unused portion remaining in the remdesivir vial.
- Transfer the required volume of reconstituted remdesivir for injection to the selected infusion bag.
- Gently invert the bag 20 times to mix the solution in the bag. Do not shake.
- The prepared diluted solution is stable for 4 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours in the refrigerator at 2°C to 8°C (36°F to 46°F).
Administration Instructions
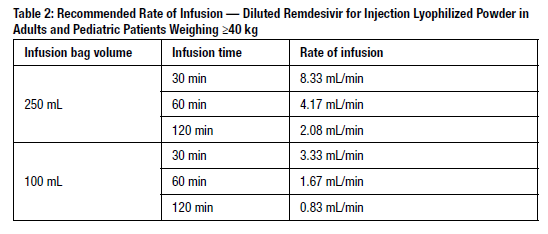
The prepared diluted solution should not be administered simultaneously with any other medication. The compatibility of remdesivir injection with IV solutions and medications other than saline is not known. Administer the diluted solution with the infusion rate described in Table 2.
Pediatric Dose Preparation and Administration
Remdesivir for Injection, 100 mg, Lyophilized Powder
For pediatric patients with bodyweight between 3.5 kg and <40 kg, use remdesivir for injection, 100 mg, lyophilized powder only.
Reconstitution Instructions
-
Remove the required number of single-dose vial(s) from storage. For each vial:
- Aseptically reconstitute remdesivir lyophilized powder by addition of 19 mL of Sterile Water for Injection using a suitably sized syringe and needle per vial.
- Discard the vial if a vacuum does not pull the sterile water for injection into the vial.
- Immediately shake the vial for 30 seconds.
- Allow the contents of the vial to settle for 2 to 3 minutes. A clear solution should result.
- If the contents of the vial are not completely dissolved, shake the vial again for 30 seconds and allow the contents to settle for 2 to 3 minutes. Repeat this procedure as necessary until the contents of the vial are completely dissolved.
- Following reconstitution, each vial contains 100 mg/20 mL (5 mg/mL) of remdesivir solution.
- Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit.
- After reconstitution, the total storage time before administration should not exceed 4 hours at room temperature or 24 hours at refrigerated temperature (2°C to 8°C [36°F to 46°F]).
Dilution Instructions
Care should be taken during admixture to prevent inadvertent microbial contamination. As there is no preservative or bacteriostatic agent present in this product, aseptic technique must be used in the preparation of the final parenteral solution. It is always recommended to administer IV medication immediately after preparation when possible.
-
Using Table 3 and Table 4, determine the volume of 0.9% saline to withdraw from the infusion bag. Table 3 and Table 4 include the volume requirements for preparing pediatric weight-based dosing regimens at 5 mg/kg and 2.5 mg/kg, respectively.
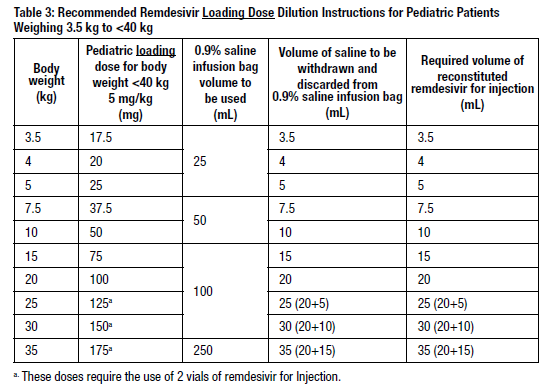
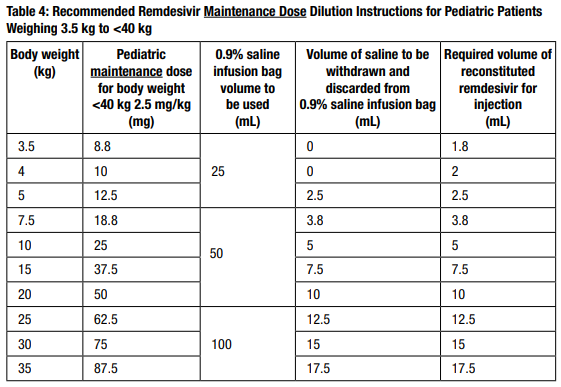
- Withdraw the required volume of saline from the bag using an appropriately sized syringe and needle. Discard the saline that was withdrawn from the bag.
- Withdraw the required volume of reconstituted remdesivir for injection from the remdesivir vial using an appropriately sized syringe per Table 3 or 4. Discard any unused portion remaining in the remdesivir vial.
- Transfer the required volume of reconstituted remdesivir for injection to the selected infusion bag.
- Gently invert the bag 20 times to mix the solution in the bag. Do not shake.
- The prepared diluted solution is stable for 4 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours in the refrigerator at 2°C to 8°C (36°F to 46°F) (including any time before dilution into intravenous infusion fluids).
Administration Instructions
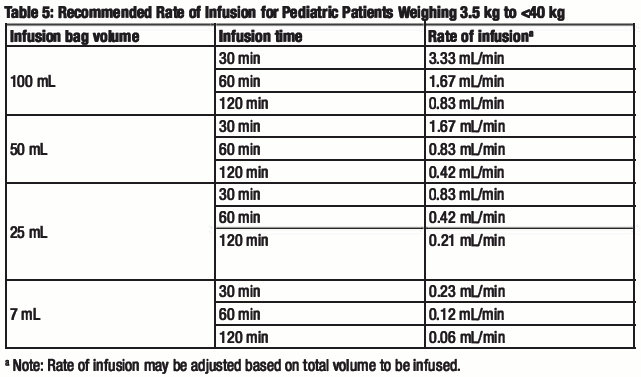
The prepared diluted solution should not be administered simultaneously with any other medication. The compatibility of remdesivir injection with IV solutions and medications other than saline is not known. Administer the diluted solution with the infusion rate described in Table 5.
Storage of Prepared Dosages
Lyophilized Powder
After reconstitution, vials can be stored up to 4 hours at room temperature (20°C to 25°C [68°F to 77°F]) before administration or 24 hours at refrigerated temperature (2°C to 8°C [36°F to 46°F]). Dilute within the same day as administration.
IMPORTANT:
This product contains no preservatives. Any unused portion of a single-dose remdesivir vial should be discarded after a diluted solution is prepared. Maintain adequate records showing the receipt, use, and disposition of remdesivir. For unused intact vials, maintain adequate records showing the disposition of remdesivir; do not discard unused intact vials.


